Wow, it's been busy. Got a new ICP mass spec with some new features not seen before on any previous ICP mass spectrometers. Now, for the first time ever, copper can be analyzed in a matrix of salt water without any matrix removal, all while keeping the detection limit below the EPA action limit. Also, no internal standard suppression more than 60%. Since we don't write papers for journal submission, I'm announcing this to the chemistry community here.
Important factors in the analysis include a specific nebuliser optimization, cones that are water cooled, but are allowed to be at a higher temperature than previous models, and a really long rinse time.
Any chemist out there that are analyzing copper in seawater on ICPMS, inquire here for smooth analyses.
Showing posts with label copper in seawater. Show all posts
Showing posts with label copper in seawater. Show all posts
Thursday, February 22, 2007
Thursday, January 04, 2007
Check out Notre Dame's ICPMS lab
This place looks pretty sweet, except for the exhaust tubes hanging all over the place. If they analyze samples for free, can I sub-contract?
 The Element 2 High-Resolution ICPMS is from the good ole boys at Thermo, who have now merged with Fisher Scientific to form a massive company that can sell you anything from post it notes to 1-Aryl-2-Pyrrolidinones. With more resolution like this, you might be able to tell the difference between 32S-33S and 65Cu. Did you know that there is a lot of sulfur in seawater, but not a lot of copper? Makes it hard to analyze for copper. Oh ya, mass 63 doesn't work either because of ArNa :)
The Element 2 High-Resolution ICPMS is from the good ole boys at Thermo, who have now merged with Fisher Scientific to form a massive company that can sell you anything from post it notes to 1-Aryl-2-Pyrrolidinones. With more resolution like this, you might be able to tell the difference between 32S-33S and 65Cu. Did you know that there is a lot of sulfur in seawater, but not a lot of copper? Makes it hard to analyze for copper. Oh ya, mass 63 doesn't work either because of ArNa :)
 The Element 2 High-Resolution ICPMS is from the good ole boys at Thermo, who have now merged with Fisher Scientific to form a massive company that can sell you anything from post it notes to 1-Aryl-2-Pyrrolidinones. With more resolution like this, you might be able to tell the difference between 32S-33S and 65Cu. Did you know that there is a lot of sulfur in seawater, but not a lot of copper? Makes it hard to analyze for copper. Oh ya, mass 63 doesn't work either because of ArNa :)
The Element 2 High-Resolution ICPMS is from the good ole boys at Thermo, who have now merged with Fisher Scientific to form a massive company that can sell you anything from post it notes to 1-Aryl-2-Pyrrolidinones. With more resolution like this, you might be able to tell the difference between 32S-33S and 65Cu. Did you know that there is a lot of sulfur in seawater, but not a lot of copper? Makes it hard to analyze for copper. Oh ya, mass 63 doesn't work either because of ArNa :)
Labels:
analytical chemistry,
copper in seawater,
ICPMS,
research
Saturday, September 16, 2006
Plumbing better than Mario and Luigi
Instead of dealing with large green pipes you have to jump into,
I have to deal with peristaltic pump tubing.
These little tubes are used to carry liquids into the mass spec. Other people use them too, such as chemists who use HPLC, IC, and SFA.
About twice a month, I have to run an analysis called FIAS or TTRA. Too make a long expanation short, the samples are mixed with buffer (pH 5.5) and internal standard (Y, In, Tb) all "online", then pushed through a column with an affinity resin that grabs the transition metals and lets the group I metals pass through. Then, the column is washed with acid, which rinses the transition metals into the mass spec which sorts them and counts them.

The way the literature describes to do this was unecessarily complicated. It explained using a sample loop and employing a water line to wash the column. It involved two valves, two pumps, four peristaltic pump lines, a double six-way valve, and finally the column.That's the way I did it and it worked for a while, but eventually it stopped working properly and I couldn't fix it. So instead of spending multiple days troubleshooting, I decided to just completely start over and re-think the whole thing.
I re-plumbed it much more simple and straight forward. Both valves, half of the double six-way valve, and the water line ended up being unnecessary. Now, loads of para-film were no longer needed to prevent leaks in the peristaltic pump tube connections. Also, less sample is used.



I have to deal with peristaltic pump tubing.

These little tubes are used to carry liquids into the mass spec. Other people use them too, such as chemists who use HPLC, IC, and SFA.
About twice a month, I have to run an analysis called FIAS or TTRA. Too make a long expanation short, the samples are mixed with buffer (pH 5.5) and internal standard (Y, In, Tb) all "online", then pushed through a column with an affinity resin that grabs the transition metals and lets the group I metals pass through. Then, the column is washed with acid, which rinses the transition metals into the mass spec which sorts them and counts them.

The way the literature describes to do this was unecessarily complicated. It explained using a sample loop and employing a water line to wash the column. It involved two valves, two pumps, four peristaltic pump lines, a double six-way valve, and finally the column.That's the way I did it and it worked for a while, but eventually it stopped working properly and I couldn't fix it. So instead of spending multiple days troubleshooting, I decided to just completely start over and re-think the whole thing.
I re-plumbed it much more simple and straight forward. Both valves, half of the double six-way valve, and the water line ended up being unnecessary. Now, loads of para-film were no longer needed to prevent leaks in the peristaltic pump tube connections. Also, less sample is used.


Tuesday, May 23, 2006
Backwards chromatography with Mass Spec
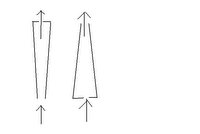
I've been trying to run the FIAS analysis for three days now, the column just doesn't seem to be doing its job. I think the problem might be that I have it backwards. I believe the way the flow should be is into the large end and out the small end. This way, the front of the column will bind the most metals, then, when the flow reverses to rinse the metals off the column with dilute acid, the metals will be at the end of the column, so they come off together.
Thursday, May 11, 2006
Copper in seawater

This is the worst analysis ever, it's called Time Transient Resolved Analysis (TTRA) or Flow Injection Analysis of Seawater (FIAS). The methods are the same, they remove the salt from the seawater so I can measure the concentration of copper. The problem is the flow, there are two pumps, two valves, four lines, a 5mL loop, column, and six way valve, and the flow has got to be steady. A simple calculation reveals 7 to the 10th ways for it to screw up.
I have to use a bunch of para-film to keep the tube joints together because the pump builds up so much pressure in the peri pump tube to push everything throught the column.
The sample flows through a tube, mixes with a buffer (the whole shabang is pH sensitive), and flows onto the column. The metals, including copper and sodium, stick to the resin in the column. The column is rinsed with water, supposedly, the sodium washes off. Then the column is rinsed with diluted nitric acid and the copper comes off and goes into the spectrometer. The metals binding strength to the iminodiacetate resin in the column depends on pH.
Subscribe to:
Posts (Atom)



